

Dilute & Concentrated
All Specific Gravities: 1.150-1.180 - 1.200-1.220 - 1.250-1.300-1.400-1.800
We offer READY TO USE Sulphuric Acid Battery electrolyte of requisite specific gravity to lead acid battery Manufacturers. This electrolyte is supplied exactly as per the required specific gravity and is something like a Just in Time ready to use product.
Sulphuric Acid Electrolyte is the most important element of lead acid storage batteries. It is diluted Sulphuric acid which undergoes electrolysis when charged. This chemical process is reversed and current is produced while discharging. The purity of an electrolyte determines performance and life of a battery.
We at Kankariya’s manufacture Sulphuric Acid electrolyte for lead acid batteries of all kinds. We have set up standardized systems using our proprietary and specially formulated manufacturing practices along with sophisticated machinery to make sound quality electrolyte conforming to various national & international standards.
We assure optimum performing levels of you battery with Kankariya's Electrolyte.


SIX Decades of Experience
We at KANKARIYA’s are a 60 year old group with the finest acid handling expertise. We are known and reputed in the market for our excellent quality of material, reliability, service and timely supply. We share a long term relationship with our customers based on trust. We are the market leaders & pioneers in our products. In all these years, with our immense On Ground experience gathered, we have developed superlative manufacturing processes and delivery systems.

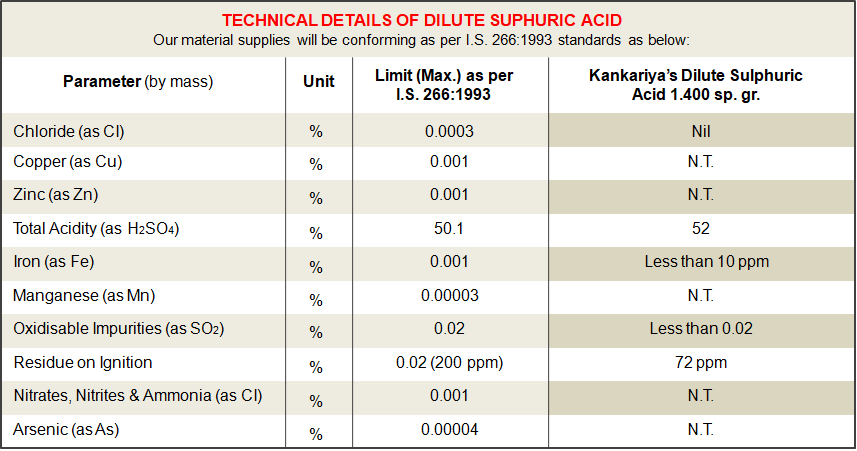
Consistent Quality, Confirming to I.S. 266:1993 standards.
Our emphasis & attitude of no compromise on Quality pushes us to deliver the best quality of material. We value our Brand image that we have maintained over the last 6 decades. We can supply you Dilute Sulphuric acid with low iron and low chlorine content that will boost up the performance of your battery. We follow the ISO 9001:2015 QMS and our systems and processes are defined to deliver the finest quality consistently.

Packaging of International Standards.
Since Sulphuric Acid transportation is highly dangerous, we ensure appropriate packaging that is sturdy and according to International (U.N.) Standards. It is our expertise to handle, package & transport Sulphuric Acid to an extent that minimizes risk. Our Export Packaging is as per UN Standards. We are also the pioneers in India to transport Dilute Sulphuric Acid in tankers. Since we transport material in our self-owned tankers, there is minimum chance of any external contamination and you get the entire material of uniform quality & specific gravity, which minimizes risks involved during transport & storage.

You Name It, We Have It.
We offer Ready to use Sulphuric Acid of requisite specific gravity to your Company. This electrolyte can be supplied exactly as per their required specific gravity and can be something like a Just in Time ready to use product. We assure optimum performing levels with Kankariya's electrolyte. If needed, we can provide these lower specific gravities like 1.100, 1.180, 1.240, 1.340, 1.300 directly too.
On Time Delivery
We at Kankariya Corporation, provide the prompt & shortest possible delivery even for huge requirements. Our set up of Bulk Sulphuric Acid storage facilities, well set infrastructure, fleet of acid tankers, skilled labour & many other features enables us for this. This can be of your advantage for bulk material needs in shortest time.

Expertise Delivered
Sulphuric Acid is a low cost but yet a tricky chemical which self contaminates itself as well as the surrounding during storage, process as well as transportation. It is a very dangerous chemical. Being in the industry for a long time and with the experience of 60 years in Acid Handling, we are amongst the finest acid handlers as we understand the product well. It is this expertise in the field of Sulphuric Acid that benefits our clients.
It has been observed with Major Battery Manufacturing Units over the last few years that they are facing huge setbacks due to Self-Dilution from Concentrated Sulphuric Acid at their factory and gradually look to switch over to procure Dilute Sulphuric Acid directly.
Buying Concentrated Sulphuric Acid of 1.840 sp gr & diluting in battery factory incurs a lot of hidden costs & quality issues which a battery company realizes in the long term. It is much advantageous in the long run in terms of cost saving and quality assurance to buy Dilute Acid of required gravity like 1.200 or 1.220 directly.
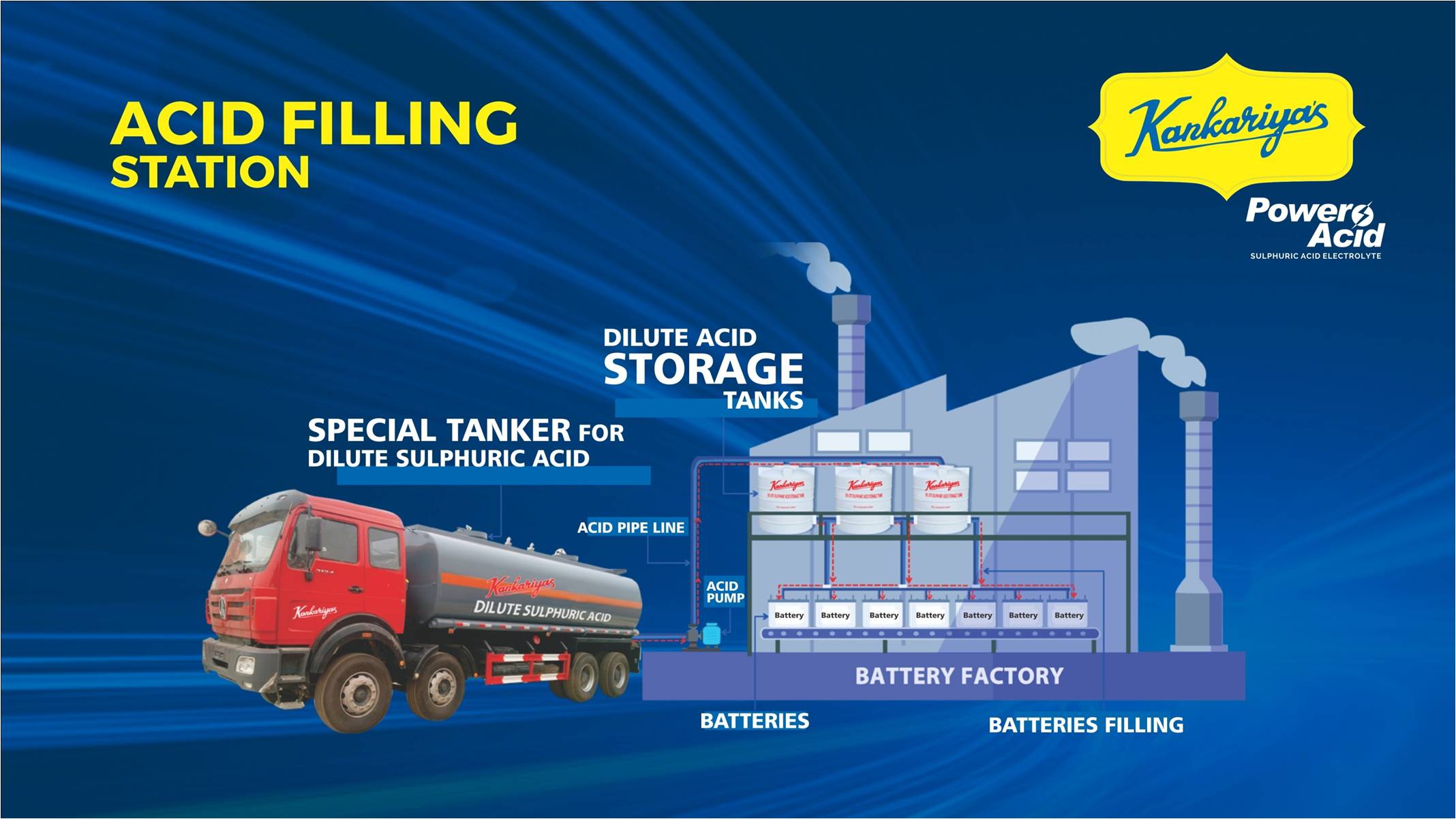
We help Install an Acid Storage & filling station in your factory which hels you to handle Sulphuric acid with ease
We help you set up an Acid filling Station at your factory to make your acid storage & handling easy.
Handling 98% Concentrated Sulphuric Acid in a factory is a headache because :
There is no wealth like knowledge, and no poverty like ignorance.
Sulphuric acid has been classified in four grades depending upon presence of impurity levels:
a) Technical (Tech)
b) Chemically pure (CP)
c) Analytical reagent (AR) or Laboratory Grade (LR)
D) Battery Grade (bg)
Concentration of Sulphuric Acid is directly proportional to its specific gravity. Some of the Concentration and specific gravities of Sulphuric acid are mentioned below :
30% 1.260
40% 1.350
50% 1.400
98% 1.840
Sulphuric Acid fumes are highly dangerous. They corrode and damage every substance, metal, electrical wires, etc. that they come in contact with. If accidently inhaled, they cause immflamation, severe burns and damage to the eyes, lungs and respiratory track, leading to health hazards. 98% Sulphuric Acid is more prone to emit fumes on a continual basis and hence proves to be a silent killer.
Sulphuric Acid and its fumes (gases) being of a highly corrosive nature, over a period of time corrodes every material in contact with it & around it like metals, its dilution machinery, pumps, pipes, electrical fittings, the factory shed, human health, etc. These losses are difficult to account for.

If sulfuric acid makes direct contact with the eyes, it can cause permanent blindness. If ingested, this chemical may cause internal burns, irreversible organ damage, and possibly death. Exposure to sulfuric acid aerosols at high concentrations leads to severe eye and respiratory tract irritation and tissue damage.

Sulphuric acid is Not flammable but highly reactive. It reacts violently with water with evolution of heat. It also react with organic materials explosively. This chemical is unique because it not only causes chemical burns, but also secondary thermal burns as a result of dehydration.
Sulphuric acid can harm crops and trees, textiles, building materials, animals, and people either as a result of exposure to long-term low concentrations or short-term high concentrations.
.png)
If in eyes:
Immediately flush eyes and skin with copious amounts of water for at least 15 to 30 minutes, holding lids apart to ensure flushing of the entire surface. Do NOT allow victim to rub eyes or keep eyes closed.
If inhaled:
Get medical aid immediately remove patient from exposure to fresh air. Administer approved oxygen supply if breathing is difficult. Administer the artificial respiration or CPR if breathing has ceased. Do NOT use mouth-to-mouth resuscitation.
.png)
If swallowed:
Do NOT induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. If spontaneous vomiting occurs, have victim lean forward with head down to avoid breathing in of vomitus. Never give anything by mouth to an unconscious person. IMMEDIATELY transport victim to an emergency facility.
.png)
Skin/clothes:
Get medical aid immediately. Immediately flush skin with copious quantities of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
a) Sulfuric acid is a highly corrosive mineral acid that challenges traditional chemical storage & handling options. Any company that stores concentrated Sulfuric acid needs to be acutely aware of the dangers that the chemical presents to your equipment and your personnel.
b) Sulfuric acid presents a serious storage challenge because it’s a very heavy chemical, especially at high concentrations. At 70-98% concentration it is nearly twice the weight of water. Being extremely heavy, it will test the mechanical integrity & strength of your storage tank.
c) Biggest challenge in working with 98% Sulfuric Acid is that it is an aggressive oxidizer. It’s also an aggressive chemical that oxidizes plastic and corrodes metals.
d) Accidental exposure to moisture or water to concentrated sulfuric acid will create a toxic aerosol or a potential explosion.
e) Highly flammable hydrogen gas can be created if Concentrated Sulfuric acid is spilled on metals.
f) 98% Concentrated Sulfuric acid can cause serious burns on the skin—even more serious than other acids.
1) Store Sulphuric Acid in an area that is cool, dry, out of direct sunlight, away from heat and ignition sources, separate from incompatible materials.
2) Do not store excess amount of Concentrated Acid in your factory for a long duration. Strictly avoid bulk storage.
3) Proper tank is crucial for safe Sulphuric Acid storage as it can impact the safety of your employees, the protection of your environment, and the cost of protecting your investment.
4) Regular inspections should be performed to ensure that your tank is maintained properly.
5) Make sure you’re cleaning your tank periodically and inspecting it visually with a flashlight, looking for any abnormalities, physical changes and signs of crystallization, damage or leaks.
6) The storing temperature of the chemicals in your tank is very important. Do not allow excess heat to be generated in the tank at all.
7) If product is transferred to another container, ensure new container is suitable for the product. Never reuse empty containers, even if they appear to be clean.
8) Store acid in an area where it is easy for people to escape in case of emergency leakage
9) Create a pit around the tank that shall contain the spilled acid and it will be contained in that area.
1) This is unwise and could cost you irreparable damage to your business.
2) Diluting Sulphuric acid should not be taken lightly. It is no cake walk. Sulphuric acid Dilution presents serious challenges that you need to be fully prepared to handle.
3) Avoid mixing or cutting chemicals in open containers or normal Plastic tanks.
4) Sulfuric acid can cause serious burns on the skin—even more serious than other acids.
5) Sulphuric Acid Fumes are silent Killers and cause irrepairable damage to surrounding area as well as serious health hazards to personnel. Thereby use of Compressed air for Dilution to create air mixing by bubbles can be very dangerous and should be avoided.
6) Use proper protective equipment while diluting acid. Avoid formation of Acid fumes.
7) Always add acid to water and never water to acid.
TAs the acid get colder it contracts and the apparent density increases and as it gets hot it expands and the apparent density decreases. Major problems occur at temperature extremes. Therefore, When taking specific gravity measurements, it is important to correct for temperature to get a true reading. As a rule of thumb, specific gravity will change by 0.007 points per 1ºC.
No. HCL or Nitric Acid should never be added to a Lead Acid Battery.
The lead-acid battery with sulfuric acid just undergoes reactions involving the lead and gives contained, nonvolatile products. By way of contrast, hydrochloric acid could be oxidized to chlorine gas at the anode and nitric acid could be reduced to nasty nitrogen oxides at the cathode. Such fumes coming from car batteries, will create damage first to the battery plates and then to the surrounding.
Using Normal water to Dilute Sulphuric Acid will hamper the performance of the battery.
This is because the Normal water will contain undesired ions and minerals like Chlorine, iron, etc which will react, thereby reducing the life of a battery.
Also, they will block the pores of the separator reducing the efficiency.
Acids can be neutralized with bases (such as sodium hydroxide, potassium hydroxide) or very basic salts (sodium carbonate, sodium bicarbonate, etc).
Battery acid used for lead acid batteries is dilute sulphuric acid & there are various situations:
1.Yes you can mix old and new acid IF you are putting tested battery grade acid received from a vendor in a storage tank which already contains battery grade acid of good quality. This happens frequently in a battery plant when acid is ordered and replenished as and when the stock diminishes.
2.Yes you can mix old and new acid IF you are replenishing new battery grade acid in a battery containing old battery grade acid. Such situations rarely crop up and are situations wherein the battery has overturned or tilted and lost acid by spilling.
3.No you cannot mix old battery acid from a scrap battery with new battery grade acid of good quality for use as fresh acid. As and when a battery becomes old the acid quality degrades with impurities leaching out from the battery plates as well as separators. In most of the cases the old drained acid when tested has been found to contain metallic impurities or other contaminants which may have got into the battery at the time of topping up with water during the service life.
Copyright © 2016 Kankariya Group. All Rights Reserved.